Hard PVD ceramic thin film coatings – What are they made of?
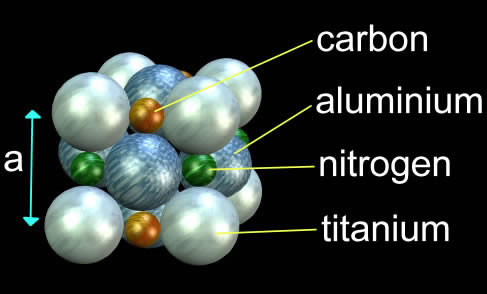
The IVth, Vth, and VIth groups of the periodic table’s carbides, nitrides, borides, and silicides are typically hard materials ideal for thin films. Metals are deposited to create the materials under an environment of nitrogen, hydrocarbons, or silicides. The surface of the substrate is where the ceramic compound is thought to form. TiAlCN, a type of titanium aluminium carbo-nitride, is an illustration of such a hard ceramic.
Because of their exceptional wear resistance, hard PVD ceramic coatings are widely employed in cutting tool applications. They can be grouped into four coating generations while having undergone constant development over time.
Hard PVD ceramic coatings – Single metal nitride PVD coatings e.g. TiN, CrN, ZrN
Simple metal nitrides like TiN, CrN, and ZrN made up the initial generation of hard PVD ceramic coatings. Due to their increased hardness compared to uncoated high speed steel or cemented carbide, these coatings have been applied commercially since the middle of the 1980s. They also have a lovely appearance, which makes them useful in decorative applications. TiN is coloured yellow-gold, CrN appears to be chromium, and ZrN is coloured green-gold. In decorative applications like watch casings, TiN, ZrN, and primarily ZrCN are used to resemble gold. These materials also serve as a durable underlayer for gold plating.
These PVD coatings are still widely used, but they don’t have enough oxidation resistance for things like high-speed machining.
For instance, TiN oxidises and degrades around 450 °C. In order to increase the working temperature range for applications like high speed machining and general high temperature wear prevention, scientists and manufacturing engineers worked on the issue.
Hard PVD ceramic coatings – Alloyed elements improve oxidation resistance, e.g. TiAlN
By depositing other elements like Cr, Al, or Y concurrently with TiN, the enhancement in oxidation resistance was made possible. Al was added, and this resulted in the stable aluminium oxide that formed on the surface and stopped further oxidation. As the temperature climbed, small amounts of chromium made the oxide denser and yttrium tended to move to grain boundaries, blocking the substrate elements’ ability to diffuse through the coating.
Hard PVD ceramic coatings – The development of superlattices
The hardness and oxidation resistance of PVD coatings were further improved, culminating in multilayers and superlattices. These thin films have many layers because they are made by alternately depositing two distinct components. When the period of the several layers is less than 100Å, multilayers are considered superlatices.
Superlattice coatings of materials with similar crystal structures tend to form columnar crystals which extend through the whole coating, provided that the thickness of the individual lamellae is sufficiently thin, typically 5–25 nm. One of the first examples of superlattice coatings was obtained by combining TiN/VN and TiN/NbN. This type of multilayered coating structure can improve the hardness, corrosion resistance, oxidation resistance as well as the toughness, compared to single layers of the same materials.
If the thickness of each lamella is thin enough, typically 5 to 25 nm, superlattice coatings of materials with similar crystal structures tend to create columnar crystals that extend through the entire coating. By mixing TiN/VN and TiN/NbN, one of the earliest examples of superlattice coatings was produced. Compared to single layers of the same materials, this form of multilayered coating structure can increase the hardness, corrosion resistance, oxidation resistance, and toughness.
Hard PVD ceramic coatings – The recent development of nanocomposite coatings
A nanocomposite coating is made up of two or more nanocrystalline phases, or two nanocrystalline phases and an amorphous phase. The fundamental concept behind the creation of nanocomposites is based on the segregation that is driven by thermodynamics in binary (ternary, quaternary) systems. This segregation causes a stable nanoscale structure to self-organize. The creation of nanocomposite PVD coatings was facilitated by the use of this general principle. Due to the well-known Hall-Petch effect, these nanometer-sized PVD coatings have improved yield strength, hardness, and toughness qualities.
Low friction coatings
Hard, wear resistant, low friction coatings such as Graphit-iC™, MoST™ and Dymon-iC™ have been developed with the automotive industry again the primary application.